Survival Analysis (Kaplan-Meier, Cox Proportional Hazards)
Survival analysis techniques are essential statistical methods used to analyze time-to-event data, particularly when dealing with censored observations. The Kaplan-Meier estimator and Cox proportional hazard model stand as the two most influential approaches in this field, empowering researchers across medical research, engineering reliability, and economic studies to draw meaningful insights from complex temporal data.
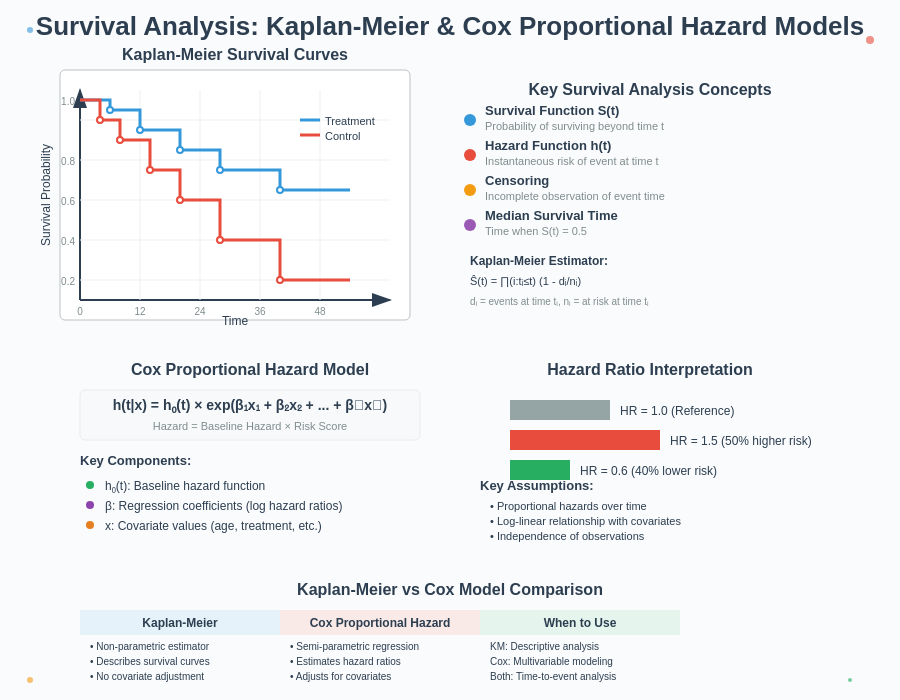
What is Survival Analysis?
Definition and Core Concepts
Survival analysis examines the expected duration until an event of interest occurs. Unlike conventional statistical methods, survival analysis uniquely handles censored data – observations where the event hasn’t occurred by the end of the study period. In clinical trials, this might represent patients who remain alive or don’t experience disease progression when the study concludes.
The fundamental components of survival analysis include:
- Survival function (S(t)): Probability of surviving beyond time t
- Hazard function (h(t)): Instantaneous rate of failure at time t
- Censoring: When complete information about survival time is unavailable
- Time-to-event data: Records when subjects experience the event of interest
Why Traditional Statistical Methods Fall Short
Standard statistical techniques prove inadequate for survival data because:
| Limitation | Explanation | Impact on Analysis |
|---|---|---|
| Censoring | Traditional methods can’t properly account for censored observations | Biased and unreliable results |
| Non-normality | Survival times are rarely normally distributed | Parametric assumptions violated |
| Time-dependent covariates | Many factors affecting survival change over time | Static models produce misleading conclusions |
| Unequal follow-up | Subjects enter and exit studies at different times | Simple averages distort true survival patterns |
The Kaplan-Meier Method Explained
What is the Kaplan-Meier Estimator?
The Kaplan-Meier estimator (also called the product-limit estimator) provides a non-parametric estimate of the survival function from lifetime data. Developed by Edward Kaplan and Paul Meier in 1958, this method has become the gold standard for visualizing and analyzing survival data.
The Kaplan-Meier curve visually represents the probability of survival over time, accounting for censored observations. Each step in the curve corresponds to one or more events (like deaths or failures).
Calculating the Kaplan-Meier Estimate
The formula for the Kaplan-Meier estimator at time t is:
S(t) = ∏ᵢ (1 – dᵢ/nᵢ)
Where:
- dᵢ = number of events at time tᵢ
- nᵢ = number of subjects at risk at time tᵢ
- ∏ represents the product of terms for all time points where tᵢ ≤ t
Interpreting Kaplan-Meier Curves
A Kaplan-Meier curve provides several key insights:
- Median survival time: The time at which the survival probability equals 0.5
- Survival probabilities: The estimated probability of surviving beyond any specific time point
- Confidence intervals: The precision of survival estimates at various time points
- Visual comparison: When plotting multiple groups, differences in survival patterns become apparent
Testing for Differences Between Groups
When comparing survival between groups (like treatment vs. control), researchers typically use:
| Test | Best Used When | Key Characteristics |
|---|---|---|
| Log-rank test | No crossing of survival curves | Tests overall difference between curves |
| Wilcoxon test | Early differences matter more | Gives more weight to early events |
| Peto test | Late differences matter more | Emphasizes events occurring later |
| Fleming-Harrington | Custom weighting needed | Allows flexible weighting of early vs. late events |
The log-rank test remains the most widely used approach, calculating the observed vs. expected events in each group under the null hypothesis of no difference.
Cox Proportional Hazard Model: Beyond Simple Comparisons
What is the Cox Proportional Hazard Model?
The Cox proportional hazard model, introduced by Sir David Cox in 1972, revolutionized survival analysis by enabling examination of how multiple variables simultaneously affect survival. Unlike fully parametric approaches, Cox’s model makes no assumptions about the shape of the baseline hazard function.
The model assumes that the hazard ratio between different strata remains constant over time – the critical “proportional hazards” assumption.
The Mathematical Framework
The Cox model expresses the hazard function as:
h(t|X) = h₀(t) × exp(β₁X₁ + β₂X₂ + … + βₚXₚ)
Where:
- h₀(t) = baseline hazard function
- X₁, X₂, …, Xₚ = predictor variables
- β₁, β₂, …, βₚ = regression coefficients
- exp(βᵢXᵢ) = hazard ratio for a one-unit change in Xᵢ
Assessing Proportional Hazard Assumption
The validity of Cox model results depends on the proportional hazards assumption. Several methods test this assumption:
- Schoenfeld residual plots: Should show random patterns without time trends
- Log-minus-log plots: Parallel lines indicate proportional hazards
- Time-dependent covariates: Non-significant interaction with time supports the assumption
- Goodness-of-fit tests: Statistical tests like the Grambsch-Therneau test
Interpreting Hazard Ratios
Hazard ratios (HR) quantify the effect of predictors on survival:
- HR > 1: Increased hazard (worse survival)
- HR = 1: No effect
- HR < 1: Decreased hazard (better survival)
For example, a hazard ratio of 1.5 for a treatment means the treatment group has a 50% higher risk of experiencing the event at any given time compared to the reference group.
Practical Applications in Research
Clinical Trials and Medical Research
Survival analysis forms the backbone of many pivotal clinical studies:
- Cancer research: Comparing progression-free survival between treatments
- Cardiovascular studies: Analyzing time to cardiac events
- Drug development: Establishing efficacy based on survival benefits
- Public health interventions: Evaluating impact on mortality rates
The landmark CONCORD programme uses these methods to compare cancer survival globally, informing health policies in numerous countries.
Engineering and Reliability Analysis
Beyond medicine, survival analysis helps engineers predict:
- Component failure rates: When parts will likely fail
- System reliability: Probability of continuous operation
- Warranty planning: Optimal coverage periods
- Maintenance scheduling: When preventive maintenance maximizes uptime
Business and Customer Analytics
Companies leverage survival methods to understand:
| Business Question | Survival Analysis Application |
|---|---|
| Customer churn | Time until a customer cancels a subscription |
| Product lifecycle | Duration until product replacement |
| Credit risk | Time until loan default |
| Employee retention | Length of employment before resignation |
Kaplan-Meier vs. Cox Model Visualization
When to Use Each Method
| Aspect | Kaplan-Meier | Cox Proportional Hazard |
|---|---|---|
| Purpose | Estimate and visualize survival | Model effect of variables on survival |
| Covariates | Limited to categorical groups | Multiple continuous or categorical variables |
| Assumptions | Non-parametric (minimal) | Proportional hazards |
| Output | Survival curve | Hazard ratios |
| Best for | Initial exploration, visualization | Multivariable analysis, effect estimation |
Complementary Roles in Analysis
Most thorough survival analyses employ both approaches:
- Begin with Kaplan-Meier curves to visualize data and identify patterns
- Use log-rank tests to detect differences between groups
- Apply Cox models to quantify effects while controlling for confounders
- Check assumptions and perform sensitivity analyses
This sequential approach provides both accessible visualizations and rigorous statistical inference.
Advanced Extensions of Survival Models
Time-Dependent Covariates
Standard Cox models assume that predictor effects remain constant, but this assumption often proves unrealistic. Time-dependent covariates allow predictors to change values throughout the study period, providing more accurate models in dynamic environments.
Consider a clinical trial where treatment dosage adjusts based on patient response. A time-dependent Cox model captures these changing treatment intensities, whereas standard models would oversimplify this crucial variation.
Implementation requires specialized data formatting with:
- Multiple rows per subject
- Start and stop times for each interval
- Updated covariate values for each time segment
- Event indicators for the final interval only
Competing Risks Analysis
Competing risks arise when subjects can experience multiple, mutually exclusive event types. For instance, in cancer studies, patients might experience:
- Cancer recurrence
- Death from cancer
- Death from other causes
Traditional Kaplan-Meier analysis treating non-events of interest as censored produces biased estimates. Instead, cumulative incidence functions (CIFs) properly account for competing events.
| Traditional Approach | Competing Risks Approach |
|---|---|
| Treats competing events as censored | Explicitly models multiple event types |
| Overestimates event probability | Produces unbiased event probabilities |
| Assumes independence between event types | Accounts for dependencies between competing events |
| Single cause-specific hazard | Subdistribution hazards for each event type |
The Fine and Gray model extends Cox regression for competing risks scenarios, modeling subdistribution hazards rather than cause-specific hazards.
Frailty Models for Clustered Data
When observations cluster within groups (like patients within hospitals or repeated measures within patients), frailty models address resulting correlation by introducing random effects.
Mathematically represented as:
h(t|X, Z) = h₀(t) × exp(βX + Z)
Where Z represents the frailty term (random effect) following distributions like gamma or normal.
Common applications include:
- Multi-center clinical trials
- Family-based genetic studies
- Recurrent event analysis
- Meta-analyses of survival data
Implementing Survival Analysis in Practice
Essential Software Tools
| Software | Strengths | Notable Packages/Functions |
|---|---|---|
| R | Most comprehensive survival analysis ecosystem | survival, survminer, cmprsk |
| Python | Integration with machine learning workflows | lifelines, scikit-survival |
| SAS | Industry standard for regulated environments | PROC LIFETEST, PROC PHREG |
| SPSS | User-friendly interface | Kaplan-Meier, Cox Regression modules |
| Stata | Elegant syntax for complex models | stcox, stcurve commands |
R’s survival package remains the gold standard, offering exceptional flexibility for both standard and advanced methods. The survminer package provides publication-quality visualizations of survival curves with minimal coding.
Common Pitfalls and Best Practices
Even experienced analysts encounter challenges with survival data:
- Violation of proportional hazards: Test this assumption and consider stratified models or time-dependent effects when violated
- Influential observations: Use dfbeta residuals to identify subjects exerting undue influence
- Collinearity among predictors: Check variance inflation factors and consider principal component analysis
- Overfitting: Follow the rule of thumb of at least 10 events per predictor variable
- Selection bias in cohort assembly: Clearly define entry criteria and consider inverse probability weighting
Sample Size and Power Considerations
Determining adequate sample size for survival studies requires specifying:
- Expected event rates in each group
- Anticipated effect size (hazard ratio)
- Desired power (typically 80-90%)
- Significance level (usually 0.05)
- Accrual and follow-up periods
- Expected dropout rate
Specialized software like PASS or the powerSurvEpi R package can calculate required sample sizes for complex survival designs.
Frequently Asked Questions
What’s the difference between Kaplan-Meier and Cox proportional hazard models?
Kaplan-Meier is a non-parametric method that estimates survival probabilities over time and works best for comparing groups, while Cox proportional hazard models allow for analyzing multiple variables simultaneously while controlling for confounders. Kaplan-Meier provides visual curves, whereas Cox models quantify effects through hazard ratios.
How do I interpret the p-value in the log-rank test?
The p-value in a log-rank test indicates whether the observed difference between survival curves could have occurred by chance. A p-value below 0.05 typically suggests a statistically significant difference between groups, meaning the treatment or factor being studied likely affects survival time.
What does “censoring” mean in survival analysis?
Censoring occurs when we don’t know the exact survival time for a subject. Right-censoring (most common) happens when the study ends before the event occurs, left-censoring when the event occurred before observation began, and interval-censoring when we only know the event occurred within a time interval. Survival analysis methods specially account for these incomplete observations.
How do I check if the proportional hazards assumption is met?
Test the proportional hazards assumption by examining Schoenfeld residuals (they should show no pattern over time), creating log-minus-log plots (should be parallel), or including time-dependent terms in your model. Statistical tests like the Grambsch-Therneau test can formally evaluate this assumption.

